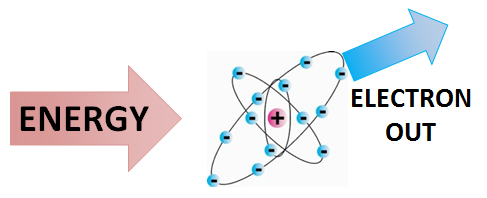
 Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. [2] If an atom or molecule gains an electron, it becomes negatively charged (an anion), and if it loses an electron, it becomes positively charged (a cation).
Ionization is the process by which ions are formed by gain or loss of an electron from an atom or molecule. [2] If an atom or molecule gains an electron, it becomes negatively charged (an anion), and if it loses an electron, it becomes positively charged (a cation).